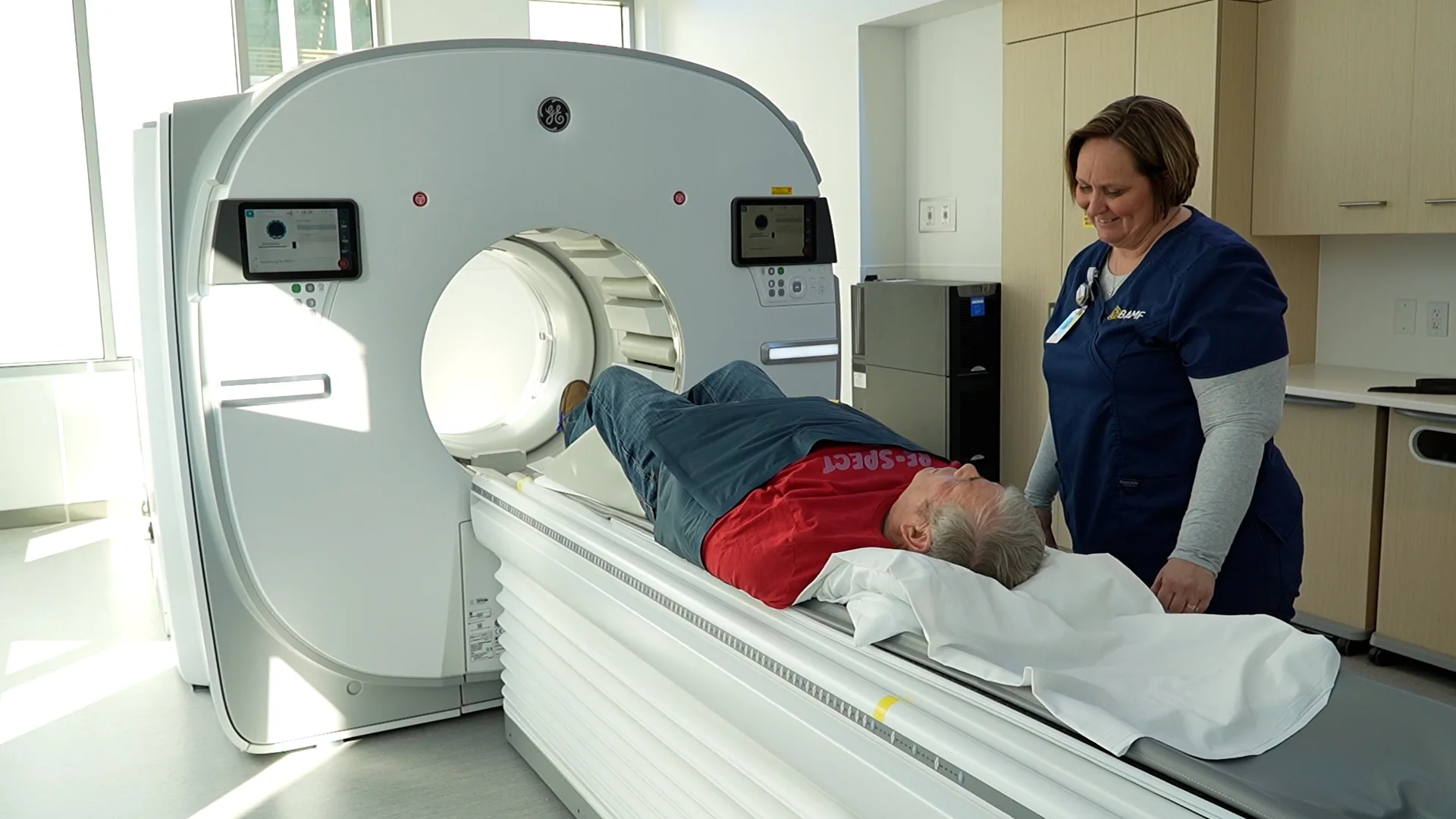
We are a top site for some of the most advanced radiopharmaceutical trials in the world. Explore innovative imaging opportunities and potentially life-saving therapies for diseases like prostate cancer, pancreatic cancer, small cell lung cancer, and many others. Clinical trial patients can expect the same exceptional care and attention as our standard treatment patients.
- Access to radiopharmaceutical imaging agents
- Access to radiopharmaceutical therapies
- Highest level of clinical safety practices
- Highest quality patient experience
- World-class Theranostics experts
- Complimentary on-site valet parking
We are currently enrolling patients for the following advanced clinical trials. If you'd like more information about which trial is best for you, complete this general interest form.
Metastatic Sarcoma | Lantheus FAPI-1301 Imaging Copy link This is a prospective Phase 1/2a study to assess safety and tolerability of 64Cu-LNTH-1363S (64Cu Radiolabeled FAPi PET/CT Imaging Agent).
This is a multicenter, open-label, prospective Phase 1/2a study to assess safety and tolerability, establish dosimetry and to identify an optimal imaging dose (radioactivity and mass dose) and imaging time window of 64Cu-LNTH-1363S (64Cu Radiolabeled FAPi PET/CT Imaging Agent) and to compare its imaging biodistribution with FAP expression by immunohistochemistry (IHC) in patients with sarcomas or GIT cancers. The study will be conducted in 2 parts (Part 1 and Part 2).
Part 1 will include 12 evaluable patients with supposed FAP-expressing solid tumors (metastatic sarcomas).
Part 1 of the study will last approximately 3 weeks for each patient and includes a Screening Period (up to 14 days), a 1-day Intervention Period, and a Safety Follow-up Period (7 days post dose).
BAMF Health is currently only enrolling to Part 1 (Metastatic Sarcoma)
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): ~32 Patients study wide
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.Gov
If you are interested in more information about this trial, please fill out this form.
Recurrent Brain Metastases from Solid Tumors | RAD101 Imaging Copy link An Open-Label, Single Dose, Single Arm, Multicenter Phase 2b Study to Establish the Imaging Performance of RAD101 Positron Emission Tomography (PET) in Participants with Suspected Recurrent Brain Metastases from Solid Tumors (GCP-PRT-101-001).
Protocol Title: An Open-Label, Single Dose, Single Arm, Multicenter Phase 2b Study to Establish the Imaging Performance of RAD101 Positron Emission Tomography (PET) in Participants with Suspected Recurrent Brain Metastases from Solid Tumors (GCP-PRT-101-001)
Sponsor: Radiopharm Theranostics Ltd.
Description: Contact BAMF for more information
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): N/A
Patient Eligibility Criteria: Contact BAMF for more information
If you are interested in more information about this trial, please fill out this form.
Pancreatic, Non-Small Cell Lung, Breast, and Colorectal | Novartis Imaging Therapy Copy link The purpose of this study is to evaluate the safety, tolerability, dosimetry and preliminary efficacy of [177Lu]Lu-NNS309 and the safety and imaging properties of [68Ga]Ga-NNS309.
Protocol Title: Phase I Open-label, Multi-center Study to Evaluate the Safety, Tolerability, Dosimetry, and Preliminary Activity of [177Lu]Lu-NNS309 in Patients With Pancreatic, Lung, Breast and Colorectal Cancers
Sponsor: Novartis Pharmaceuticals
Description: The purpose of this study is to evaluate the safety, tolerability, dosimetry and preliminary efficacy of [177Lu]Lu-NNS309 and the safety and imaging properties of [68Ga]Ga-NNS309 in patients aged ≥ 18 years with locally advanced or metastatic pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC), HR+/HER2- ductal and lobular breast cancer (BC), triple negative breast cancer (TNBC) and colorectal cancer (CRC).
The study will be done in two parts. The first part is called “escalation” and the second part is called “expansion”. In both parts of the study, patients will initially be imaged with a [68Ga]Ga-NNS309 positron emission tomography (PET)/ computed tomography (CT) or PET/magnetic resonance imaging (MRI) scan and will be evaluated for eligibility for [177Lu]Lu-NNS309 treatment. In the escalation part, different doses of [177Lu]Lu-NNS309 will then be tested to identify recommended dose(s) (RD(s)) for further evaluation. The expansion part of the study will examine the safety and preliminary efficacy of [177Lu]Lu-NNS309 at the RD(s) determined during the escalation part. The end of study will occur when at least 80% of the patients per disease group in the expansion part have completed the follow-up for disease progression or discontinued from the study for any reason, and all patients have completed treatment and the 36-month long-term follow-up period.
Patient Enrollment at BAMF: Early November 2024
Number of patients to be enrolled (study wide): 124
Patient Eligibility Criteria: please click here to see the complete list at ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Liver Cancer | RayzeBio, RYZ801-101 Imaging Therapy Copy link A single arm, open-label Phase 1/1b study of the theranostic pair RYZ811 (diagnostic) and RYZ801 (therapeutic) to identify and treat subjects with GPC3+ unresectable HCC.
Protocol Title: A single arm, open-label Phase 1/1b study of the theranostic pair RYZ811 (diagnostic) and RYZ801 (therapeutic) to identify and treat subjects with GPC3+ unresectable HCC
Sponsor: RayzeBio, Inc.
Description: To determine the recommended phase 2 dose (RP2D) of RYZ801 in subjects with GPC3+ unresectable HCC (Dose Escalation only). To assess the safety and tolerability of RYZ801 in subjects with GPC3+ unresectable HCC. Safety and tolerability of RYZ801 as measured by incidence and severity of AEs, including SAEs, laboratory changes, and other safety findings. To assess the safety and tolerability of RYZ811 in subjects with unresectable HCC. Safety and tolerability of RYZ811 as measured by incidence and severity of AEs, including SAEs, laboratory changes, and other safety findings. To evaluate the biodistribution of RYZ811 in subjects with unresectable HCC. SUV (mean, maximum and peak) of organs and tumors; volume of RYZ811 avid uptake in tumor lesions; and tumor-to-normal organ tracer uptake ratios.
Key Inclusion Criteria:
Age of at least 18 years at the time of signing the informed consent form (ICF)
Histologically/cytologically confirmed diagnosis of HCC.
At least 1 prior systemic therapy for unresectable HCC
Key Exclusion Criteria:
Subjects with fibrolamellar carcinoma, sarcomatoid HCC or combined hepatocellular cholangiocarcinoma
Prior liver transplantation or candidates for liver transplantation
Documented hepatic encephalopathy
Prior EBRT to the liver
Prior liver radioembolization
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): ~70
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Neuroendocrine Tumors | Perspective Therapeutics Therapy Copy link Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumors
Title: A Phase I/IIa First-in-Human Study of [212Pb]VMT-α-NET Targeted Alpha-Particle Therapy for Advanced SSTR2 Positive Neuroendocrine Tumors
Sponsor: Perspective Therapeutics
Description: This is a prospective, multi-center open-label dose escalation, dose expansion study of [212Pb]VMT-α-NET in up to 160 adult subjects with unresectable or metastatic SSTR2-expressing neuroendocrine tumors (NETs) who have not received prior peptide receptor radionuclide therapy (PRRT).
The radioactivity dose escalation period (Phase I) tests up to 4 escalating radioactivity dose cohorts of up to 8 subjects (administered at approximately 8-week intervals) at the assigned cohort radioactivity dose.
Pre-specified dose adjustments and individual stopping rules for repeat treatment cycles are based on observed dose-limiting toxicities (DLTs) and adverse events (AEs).
Additionally, up to 40 subjects may be enrolled in each of the cohorts.
The Maximum Tolerated Dose (MTD) will be determined based on observed DLTs within 42 days of the first treatment cycle.
The recommended expansion (Phase IIa) dose(s) will be determined following a holistic analysis of observed DLTs, AEs, estimated cumulative organ radiation exposure, and efficacy signals over the course of all treatment cycles for all dose cohorts.
If MTD can not be identified within the 4 radioactivity dose cohorts, a Maximum Feasible Dose (MFD), incorporating manufacturing and logistical considerations for [212Pb]VMT-α-NET production, may be determined.
Up to 120 subjects will be considered for enrollment in the dose-expansion phase (Phase IIa) with approximately 100 subjects with GEP-NETs, approximately 10
subjects with bronchial NETs [small cell lung cancer], and approximately 10 subjects with pheochromocytoma or paragangliomas)
Reno-protective amino acids will be co-administered in a separate IV line prior to each [212Pb]VMT-α-NET dose in all subjects. Escalation will be based on a modified toxicity probability interval design [mTPI-2] until MTD is identified or the pre-specified rules are met.
A lead-in dosimetry sub-study will be conducted during the dose escalation period in which all subjects in the first two dose cohorts will undergo dosimetric evaluation prior to receiving the therapeutic agent.
Patient Enrollment at BAMF: recruiting now
Number of patients to be enrolled (study wide): 280
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Pancreatic Cancer | NMK89P101 Trial Imaging Copy link Assess safety, tolerability, pharmacokinetics, radiation dosimetry, and positron emission tomography (PET) imaging properties of 89Zr-labeled hNd2
Title: A Phase I trial to assess safety, tolerability, pharmacokinetics, radiation dosimetry, and positron emission tomography (PET) imaging properties of 89Zr-labeled hNd2 (NMK89) in patients with pancreatic cancer histologically positive for MUC5AC
Short Name: NMK89P101
Sponsor: Nihon Medi-Physics
Number of patients to be enrolled study wide: 10
Patient enrollment at BAMF: recruiting now
Patient eligibility criteria: Please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form
Prostate Cancer Imaging | Lantheus MIRROR Imaging Copy link Prostate Cancer Imaging | Lantheus MIRROR
Protocol Title: A Phase 4 Open-Label Multicenter Study of PYLARIFY® PET/CT or PET/MRI in Men With Newly Diagnosed Favorable Intermediate Risk (FIR) Prostate Cancer
Sponsor: Lantheus Medical Imaging
Description: The purpose of this study is to learn whether PYLARIFY® PET imaging (study scan) can safely and accurately detect the presence or absence of prostate cancer growing beyond the prostate gland in men with favorable intermediate risk prostate cancer.
Participants will receive a single dose of PYLARIFY® injection followed by a single whole-body PET/CT or PET/MRI scan acquired at 1 to 2 hours after PYLARIFY injection. Participants with positive study scan results that are suspicious for prostate cancer outside of the prostate gland may be asked to undergo additional diagnostic test(s) and/or recommend certain treatment(s) for prostate cancer within 2 to 90 days after the study scan. Participants will be monitored for up to 12 months to collecting information about treatment they receive for prostate cancer and results of regular PSA blood draws if ordered by doctors for up to 12 months after the study scan.
Patient Enrollment at BAMF: Open to enrollment
Number of patients to be enrolled (study wide): 274
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Prostate Cancer | Fusion FPI-2265 Therapy Copy link Prostate Cancer | Fusion Pharmaceuticals, AlphaBreak
Protocol Title: A Phase 2/3, Randomized, Open-Label, Multicenter Study to Evaluate the Safety and Efficacy of FPI-2265 (225Ac-PSMA-I&T) in Patients With PSMA-Positive Metastatic Castration-Resistant Prostate Cancer (mCRPC), Previously Treated With 177Lu-PSMA Radioligand Therapy (RLT)
Sponsor: Fusion Pharmaceuticals Inc.
Description: This is an open-label, randomized, multicenter study of FPI-2265 (225Ac-PSMA-I&T). The dose optimization Phase 2 part will be investigating the safety, tolerability, and anti-tumor activity of novel dosing regimens of FPI-2265 in participants with PSMA-positive mCRPC who have been previously treated with 177Lu-PSMA-617 or another 177Lu-PSMA radioligand therapy (RLT).
The purpose of the dose optimization segment (Phase 2) is to determine the recommended FPI-2265 dose and regimen. Conclusions from Phase 2 will be based on safety, tolerability, and anti-tumor activity.
Participants with PSMA positive scans will be randomized (1:1:1) to one of three different dosing arms:
Arm 1: Will consist of nine doses of FPI-2265, administered every four weeks at 50 kBq/kg.
Arm 2: Will consist of six doses of FPI-2265, administered every six weeks at 75 kBq/kg.
Arm 3: Will consist of four doses of FPI-2265, administered every eight weeks at 100 kBq/kg.
Participants will be monitored and assessed for efficacy response, disease progression and adverse events.
Patient Enrollment at BAMF: Open to enrollment
Number of patients to be enrolled (study wide): 60
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Prostate Cancer | Novartis PSMA Delayed Castration Therapy Copy link An Open-label Study Comparing Lutetium (177Lu) Vipivotide Tetraxetan Versus Observation in PSMA Positive OMPC (PSMA-DC)
Protocol Title: An Open-label Study Comparing Lutetium (177Lu) Vipivotide Tetraxetan Versus Observation in PSMA Positive OMPC (PSMA-DC)
Sponsor: Novartis Pharmaceuticals
Description: The purpose of this study is to evaluate the efficacy and safety of lutetium (177Lu) vipivotide tetraxetan (AAA617) in participants with oligometastatic prostate cancer (OMPC) progressing after definitive therapy to their primary tumor. The data generated from this study will provide evidence for the treatment of AAA617 in early-stage prostate cancer patients to control recurrent tumor from progressing to fatal metastatic disease while preserving quality of life by delaying treatment with androgen deprivation therapy (ADT).
All participants will be assessed for eligibility and will undergo baseline disease assessments including a mandatory gallium (68Ga) gozetotide (also known as [68Ga]Ga-PSMA-11) or piflufolastat (18F) (also known as [18F]DCFPyL) PET/CT scan and conventional imaging (i.e., CT/MRI and bone scans). Piflufolastat (18F) PET/CT scan will be performed in countries where it is approved. Stereotactic Body Radiation Therapy (SBRT) will be administered to all metastatic Prostate Cancer (PC) lesions after randomization and before the start of treatment with AAA617 or observation.
- The duration of SBRT procedures is approximately 3 weeks.
- For participants randomized to the investigational arm (AAA617), the treatment duration will be up to 4 cycles of AAA617. For participants randomized to the control arm (observation) the treatment duration will end at the last fraction of SBRT administration.
- The visit frequency will be every week 1 and 3 of each of the 4 cycles and every 16 weeks thereafter (for both arms) until first event of disease progression (RECIST 1.1)
- The study duration is approximately 6.5 years.
Patient Enrollment at BAMF: Open to Enrollment
Number of patients to be enrolled (study wide): 450
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
Small Cell Lung Cancer | RYZ101-101 Therapy Copy link Small Cell Lung Cancer | RYZ101-101
Protocol Title: Study of RYZ101 in Combination With SoC in Subjects With SSTR+ ES-SCLC
Sponsor: RayzeBio, Inc.
Description: This Phase 1b study aims to determine the safety, preliminary antitumor activity, and pharmacokinetics (PK) of RYZ101 in combination with standard of care (SoC) therapy consisting of carboplatin + etoposide + atezolizumab in untreated subjects with somatostatin receptor expressing (SSTR+) ES-SCLC.
Patient Enrollment at BAMF*: Open to enrollment
Number of patients to be enrolled (study wide): 31
Patient Eligibility Criteria: please click here to see the complete list on ClinicalTrials.gov
If you are interested in more information about this trial, please fill out this form.
*This trial is conducted in collaboration with Corewell Health™. If you complete the interest form, we may provide your information to their staff
Clinical Trial Updates
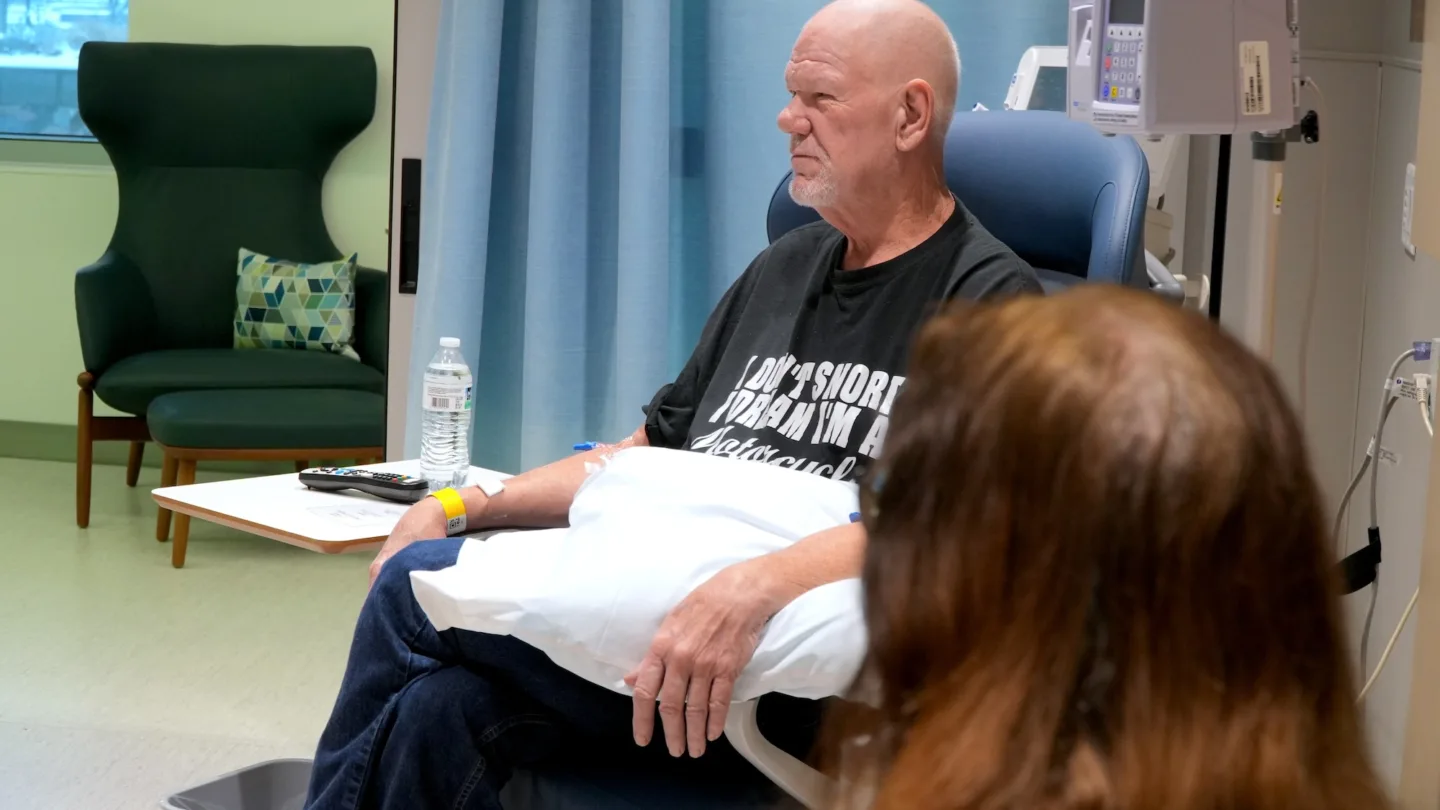
Fueled by Hope and the Open Road
David was the first person in the United States to receive a new experimental agent to treat pancreatic cancer.
January 22, 2025
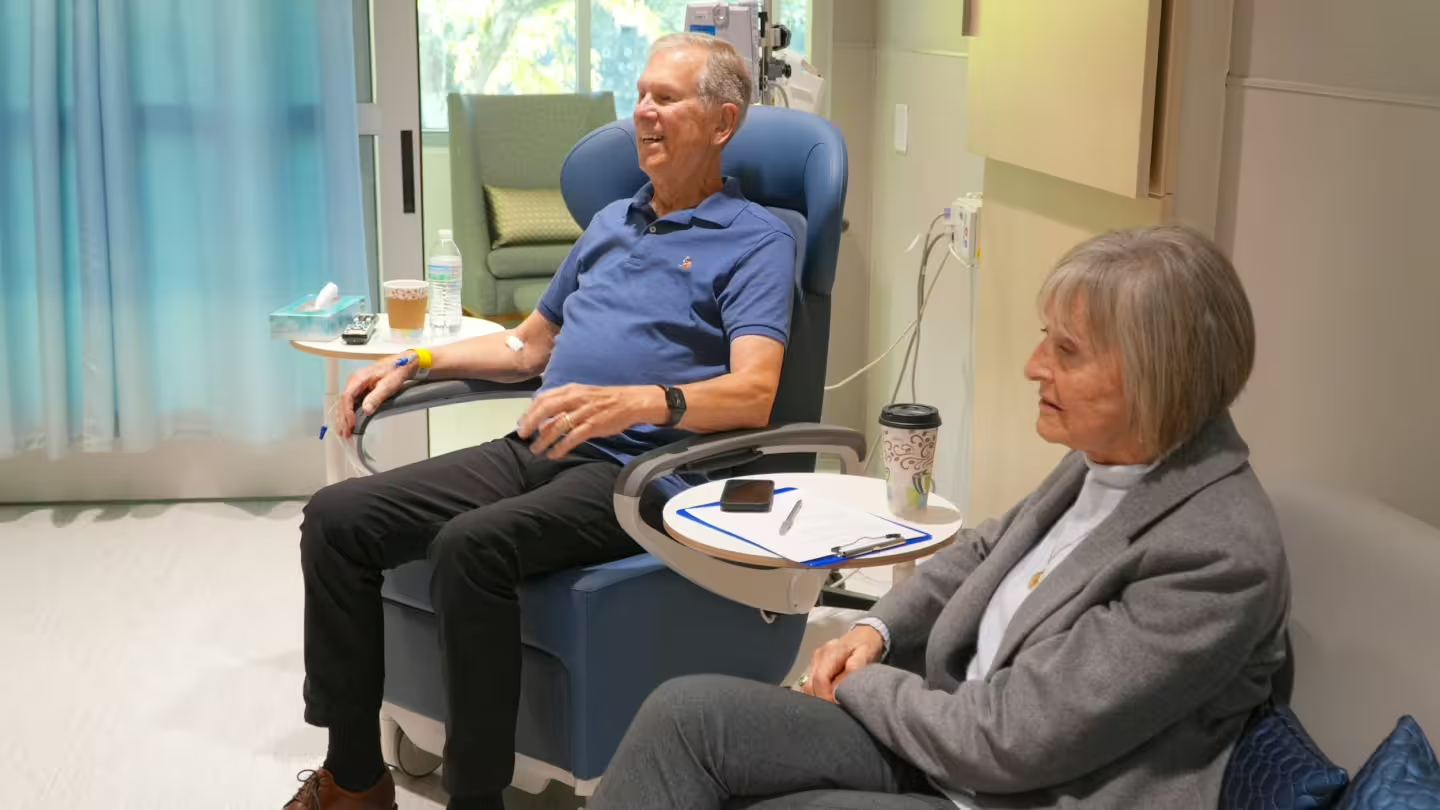
“Make Damn Sure You Get a PSA Test”
Jan says he had run out of treatment options for his metastatic prostate cancer, but what he heard when he came to BAMF was hope.
October 29, 2024

Radiopharm Theranostics and BAMF Health Announce Strategic Collaboration to Manufacture and Dose 18F-RAD 101 for Phase 2b Imaging Study of Brain Metastasis
BAMF Health will be a site for Radiopharma Theranostics’ clinical trial and will also be manufacturing 18F-RAD 101 for other trial sites.
October 21, 2024