“We are proud to pioneer the first U.S. clinical trial of RAD101,” said Harshad R. Kulkarni, MD, Chief Medical Advisor at BAMF Health and Principal Investigator of this Phase 2b study. “This marks an important step toward improving diagnostic precision and enabling more evidence-based, individualized treatment decisions for patients with brain metastases following stereotactic radiosurgery.”
The U.S. multicenter, open-label, single arm Phase 2b clinical trial1 is evaluating the diagnostic performance of 18F-RAD101 in 30 individuals with confirmed recurrent brain metastases from solid tumors of different origins. The primary objective of the study is concordance between 18F-RAD101 positive lesions and those seen in conventional imaging (MRI with gadolinium) in participants with suspected recurrent brain metastases.
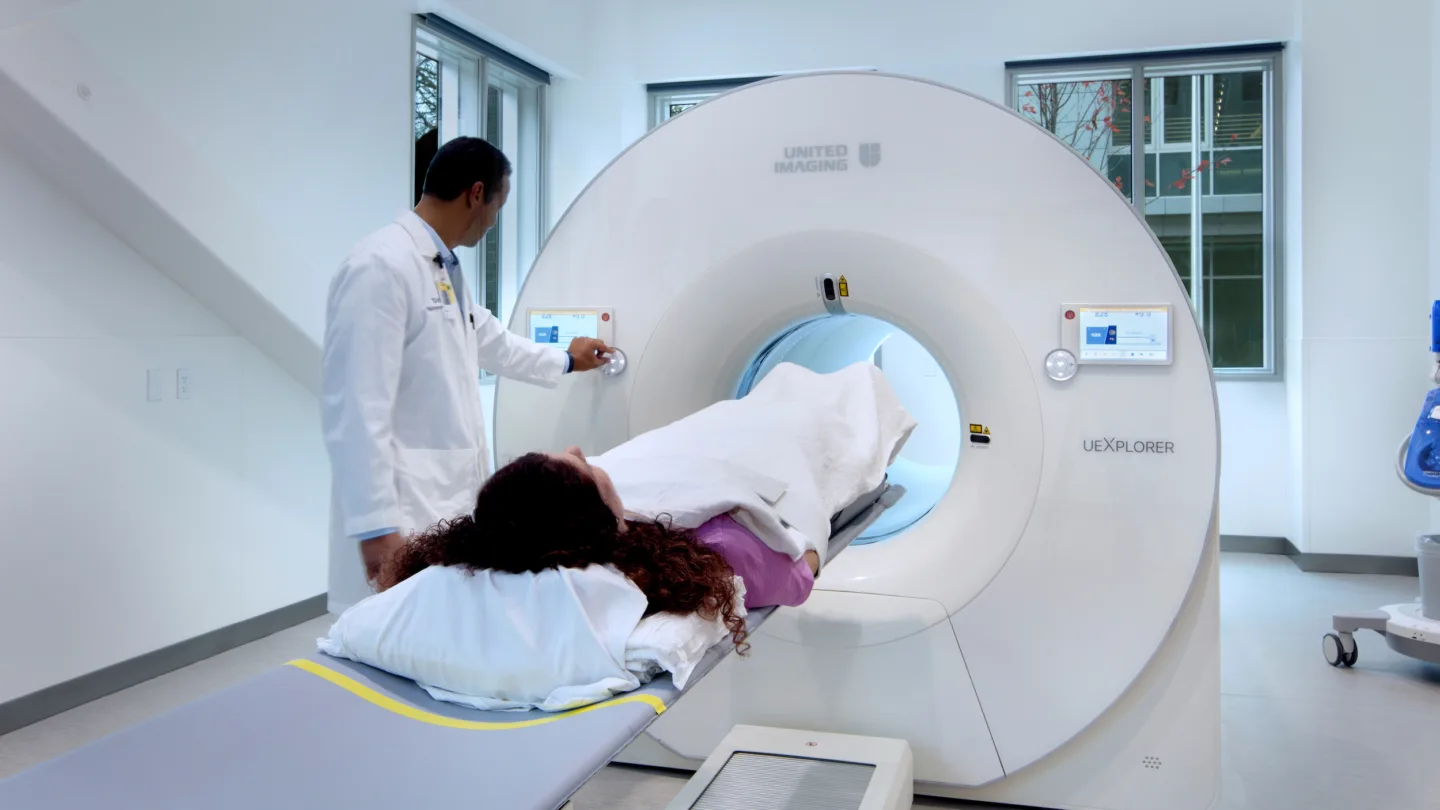
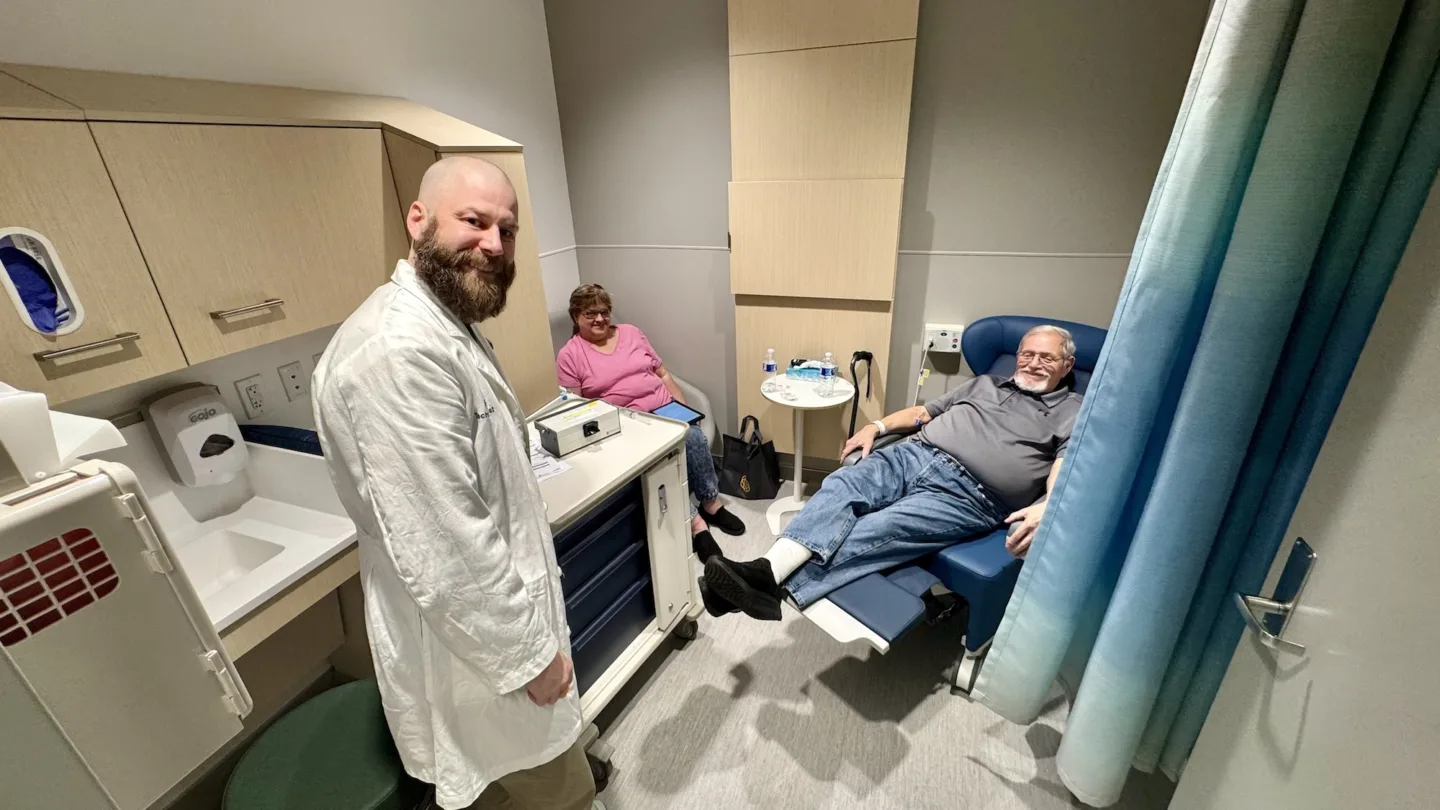
“This trial is an excellent illustration of BAMF Health’s clinical trials platform in action,” added BAMF Health’s Director of Clinical Trials. “Our Radiopharmacy is producing the imaging agent on-site, our clinic team is caring for the patient and providing the best image in the world, and our clinical trials team expertly coordinates it all. BAMF’s facility and team were built to do trials just like this.”