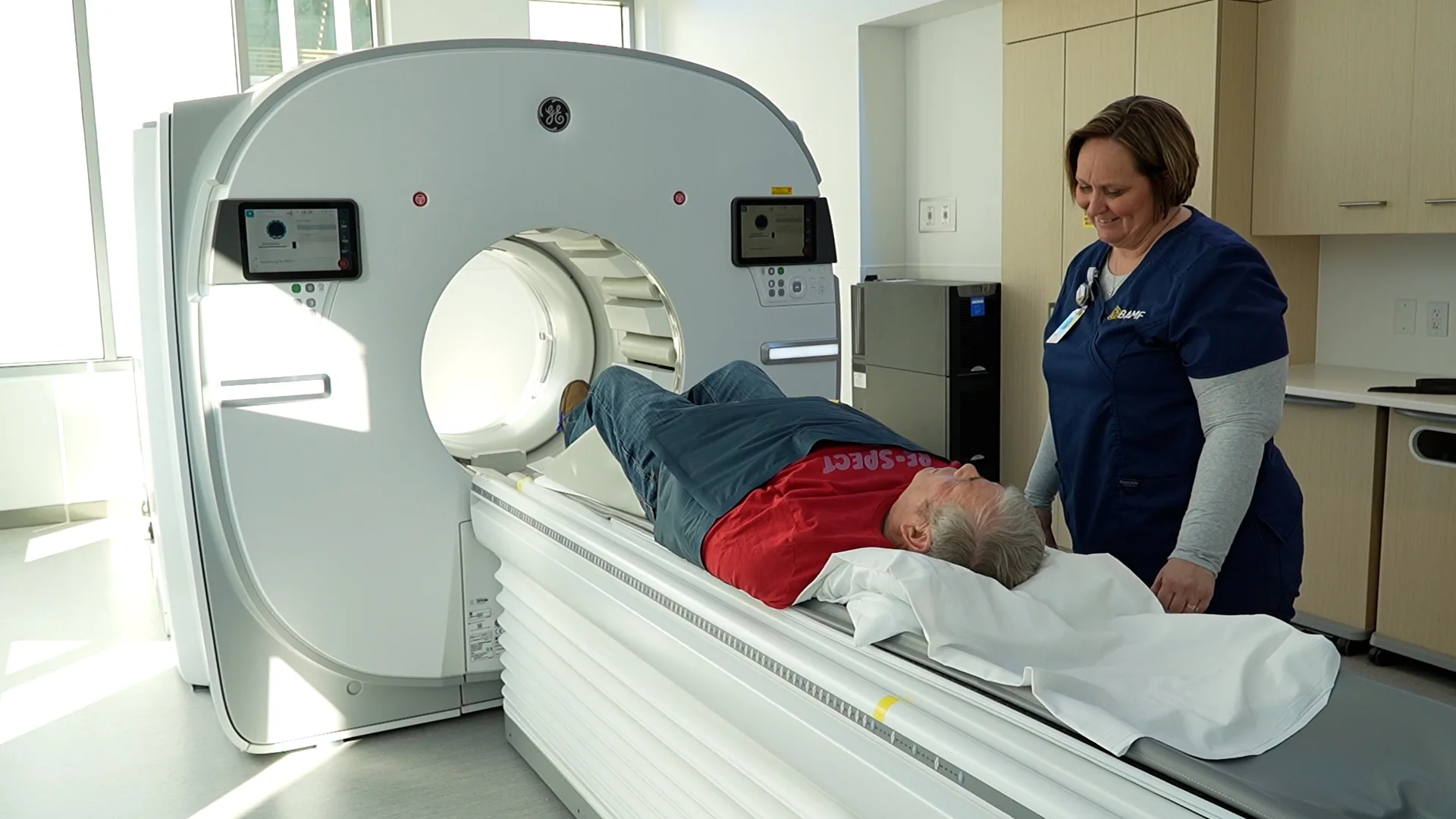
We are a top site for some of the most advanced radiopharmaceutical trials in the world. Explore innovative imaging opportunities and potentially life-saving therapies for diseases like prostate cancer, pancreatic cancer, small cell lung cancer, and many others. Clinical trial patients can expect the same exceptional care and attention as our standard treatment patients.
- Access to radiopharmaceutical imaging agents
- Access to radiopharmaceutical therapies
- Highest level of clinical safety practices
- Highest quality patient experience
- World-class Theranostics experts
- Complimentary on-site valet parking
BAMF Health is working every day to conduct additional clinical trials to improve disease management using molecular imaging and molecularly targeted radiation therapy.
Clinical Trial General Interest Copy link
If you are interested in learning more about participating in a clinical trial, please fill out this form.
Metastatic Sarcoma | Lantheus FAPI-1301 Copy link
This is a multicenter, open-label, prospective Phase 1/2a study to assess safety and tolerability, establish dosimetry and to identify an optimal imaging dose (radioactivity and mass dose) and imaging time window of 64Cu-LNTH-1363S (64Cu Radiolabeled FAPi PET/CT Imaging Agent) and to compare its imaging biodistribution with FAP expression by immunohistochemistry (IHC) in patients with sarcomas or GIT cancers. The study will be conducted in 2 parts (Part 1 and Part 2).
Part 1 will include 12 evaluable patients with supposed FAP-expressing solid tumors (metastatic sarcomas).
Part 1 of the study will last approximately 3 weeks for each patient and includes a Screening Period (up to 14 days), a 1-day Intervention Period, and a Safety Follow-up Period (7 days post dose).
BAMF Health is currently only enrolling to Part 1 (Metastatic Sarcoma)
Patient Enrollment at BAMF: Actively enrolling
Number of patients to be enrolled (study wide): ~32 Patients study wide
Patient Eligibility Criteria: Please click here to see the complete list at ClinicalTrials.Gov
Clinical Trial Updates
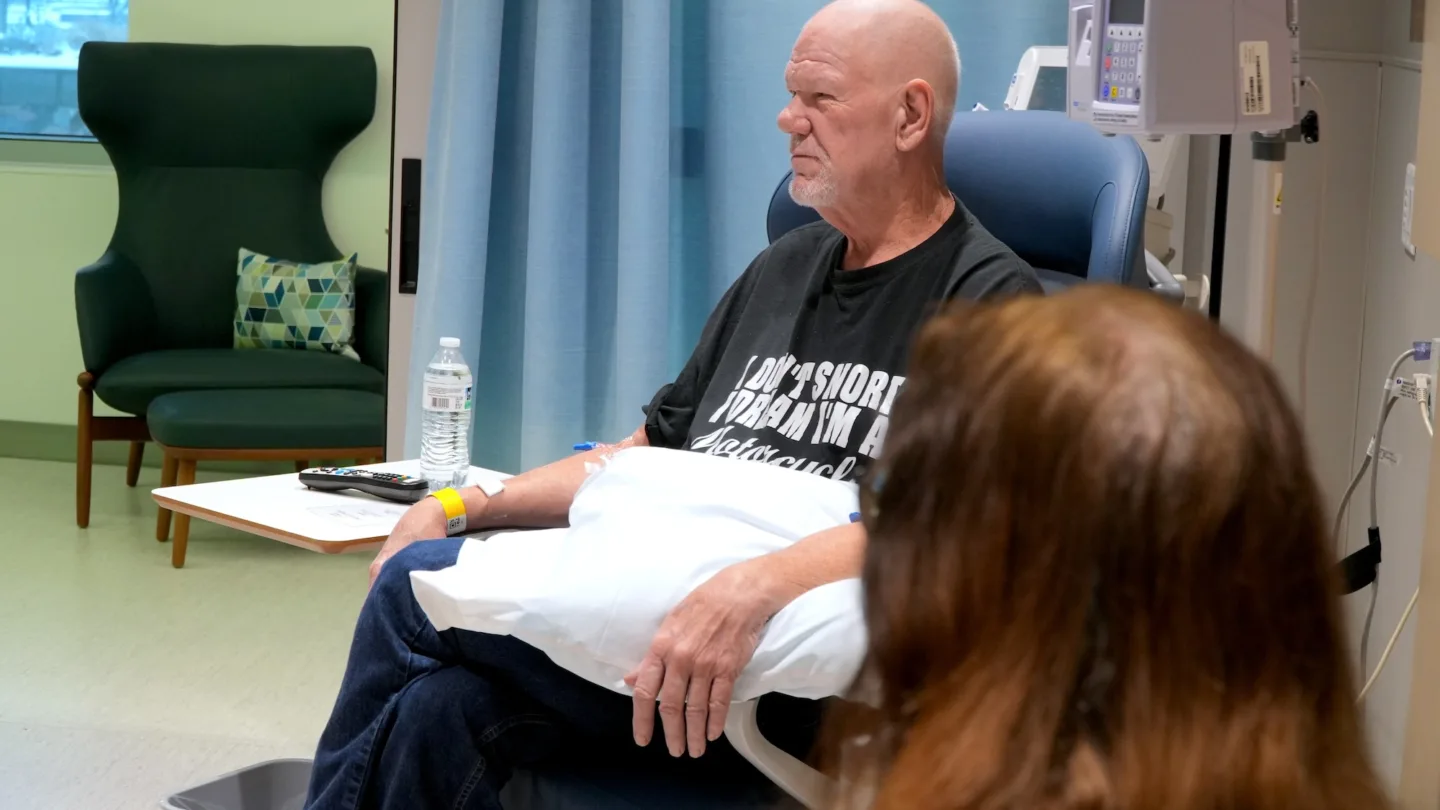
Fueled by Hope and the Open Road
David was the first person in the United States to receive a new experimental agent to treat pancreatic cancer.
January 22, 2025
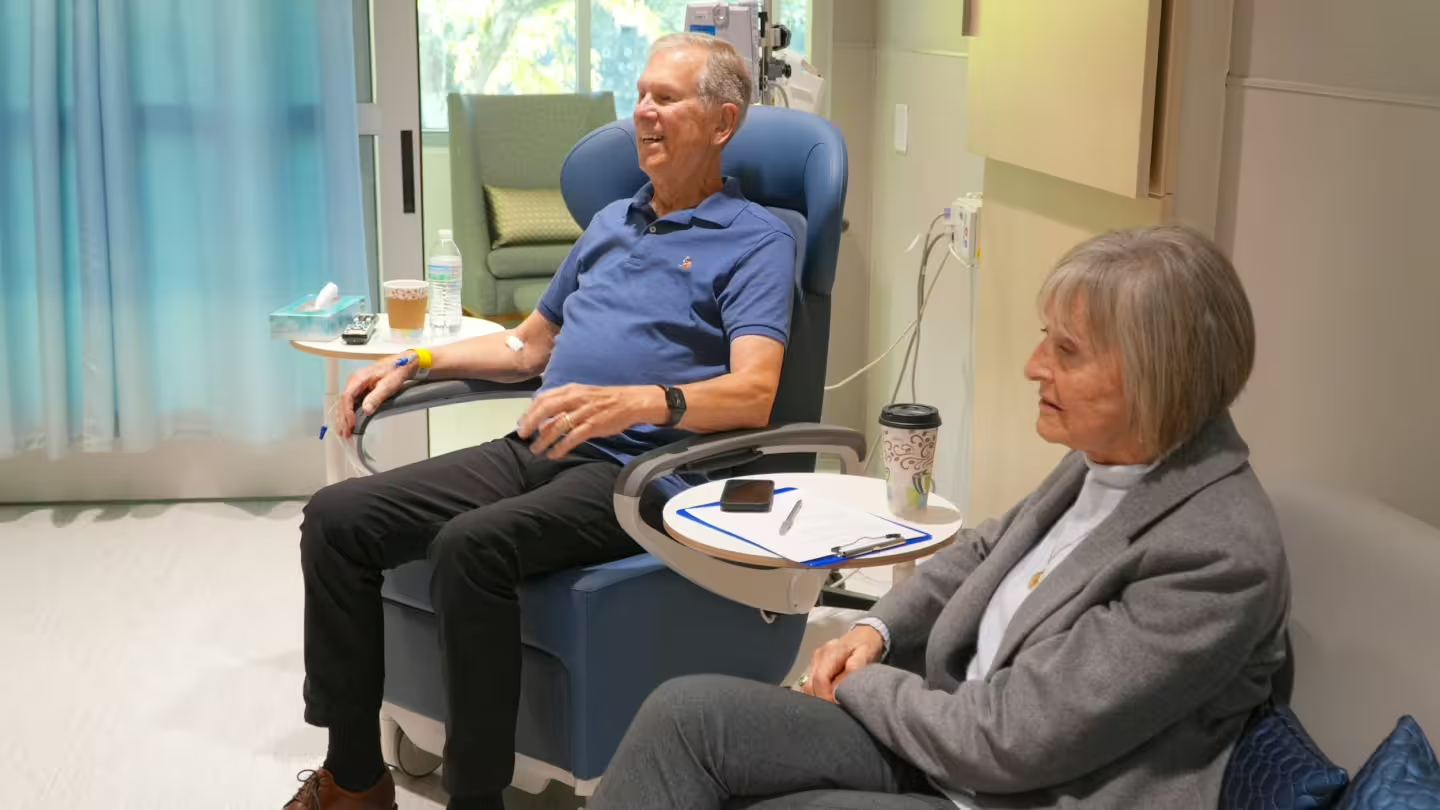
“Make Damn Sure You Get a PSA Test”
Jan says he had run out of treatment options for his metastatic prostate cancer, but what he heard when he came to BAMF was hope.
October 29, 2024

Radiopharm Theranostics and BAMF Health Announce Strategic Collaboration to Manufacture and Dose 18F-RAD 101 for Phase 2b Imaging Study of Brain Metastasis
BAMF Health will be a site for Radiopharma Theranostics’ clinical trial and will also be manufacturing 18F-RAD 101 for other trial sites.
October 21, 2024